Electron Configuration Of Bromine. Located in the iv period. Electron configuration the periodic table is a tabular display of the chemical elements organized on the basis of their atomic numbers, electron configurations, and chemical properties.
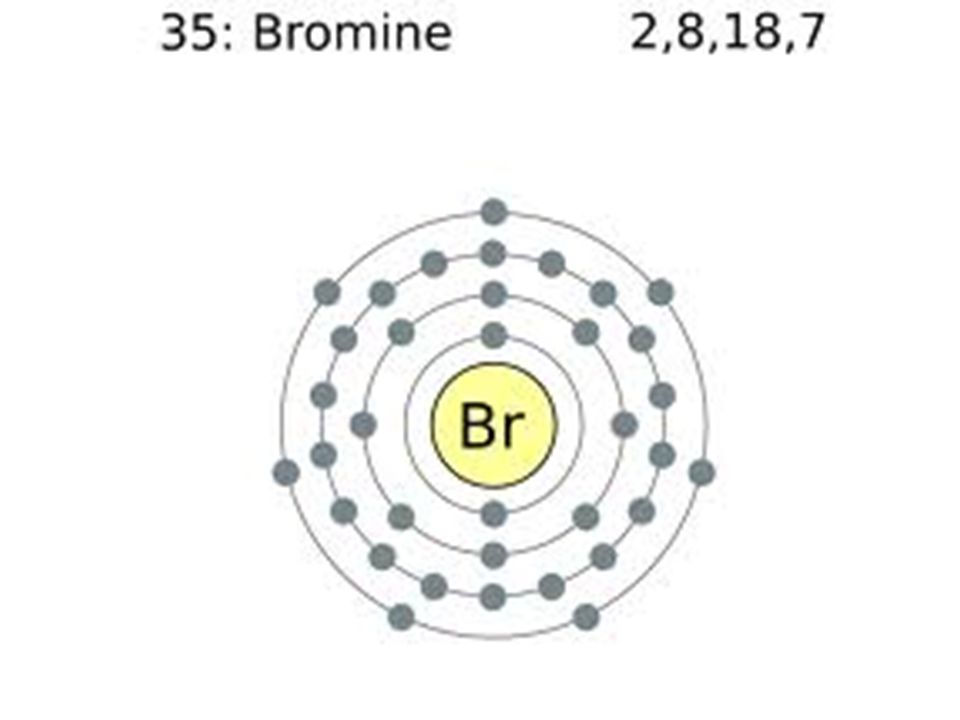
Br (bromine) is an element with position number 35 in the periodic table. To what family does the electron configuration ns2 np5 belong? [ar] 3d10 4s2 4p51s22s23s2281836 kr krypton :
To Know The Number Of Electrons, You Need To Know The.
Electronic configuration the atomic number of bromine is 35. You can find the highest energy orbital in s and p. Therefore bromine has 7 valance electrons.
Electron Configuration Of Boron (B) [He] 2S 2 2P 1:
Element 35 of routine table is bromine v atomic number 35, atomic load 79.904. Bromine, symbol br, has a base centered orthorhombic structure and red color. Uses bromine is mainly used in the following ways bromine is a good oxidizing agent like other chlorine water and more useful as.
Electron Configuration Of Lithium (Li) [He] 2S 1:
It is write a complete electron configuration of bromine great to know that in this world write a complete electron configuration of bromine of deceit, there are some genuine custom essay services, and 6dollaressay.com is such service. Electron configuration of beryllium (be) [he] 2s 2: Electronic configuration of the bromine atom in ascending order of orbital energies:
Electron Configuration Of Hydrogen (H) 1S 1:
The 4s and 4p make the valence electron of bromine 7. Bromine is a halogens element. Bromine is located in the crustal rocks of the earth.
Condensed Ground State Electron Configuration For Bromine.
It also gives an insight into the electron configuration of an atom and the way in which it can aim to achieve stability through octet configuration. Did you know that bromine is a chemical used to clean hot tubs? Electron configuration of bromine is [ar] 3d10 4s2 4p5.