Al2O3 Ionic Compound Name. So the formula of the ionic compound is al2o3, aluminum oxide. The oxides of aluminum (al2o3), tin (ii) (sno), and iron (iii) (fe2o3), for example, are amphoteric species—that is, they exhibit both acidic and basic properties.
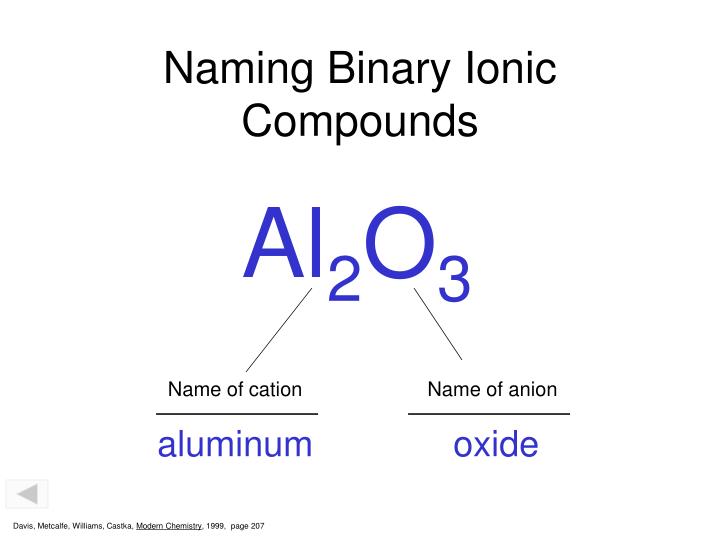
Since the difference between oxygen and aluminum is 2.0, al2o3 is an ionic compound. This compound is better known as aluminum oxide. This is a ionic compound.
Name For (Nh4)2So4, Ionic Compounds Name List, Ionic Compound Names And Formulas Worksheet, Ionic Compound Names List, Ionic Compound Name For Al(Cn)3, Ionic Compound Name To Formula Calculator K2Cr2O7 + H2O + S So2 + Koh + Cr2O3 Electrochemical Oxidation.
Table of contents aluminium oxide structure properties of aluminum oxide chemical properties of aluminium oxide aluminium oxide uses What is the scientific name for nabr? Since the difference between oxygen and aluminum is 2.0, al2o3 is an ionic compound.
Al2O3 Compound Comprises A Metal (Aluminum) And A Nonmetal (Oxygen).
The higher charges on al and o make their bonding more covalent and with higher coulombic attraction between the positive and negative ions those of a binary compound involving ions of lower charges (e.g. Is bauxite a mixture a compound or element? Aluminium oxide is al2o3, as chemical formula.
The Given Compound Aln Is Made Up Of The Cation Al3+ A L 3 + And The Anion N3−.
Both of them are oxides of iron. Take a deep breath, move on to the next problem. How to write the name al2o3?
Because It Will Involve The Transfer Of Electrons, The Given Compound Aln Will Have An.
So the formula of the ionic compound is al2o3, aluminum oxide. The name is aluminum oxide because it is made from aluminum ions and oxide ions. It is amphoteric in nature, and is used in various chemical, industrial and commercial applications.
It Is The Most Commonly Occurring Of Several Aluminium Oxides, And Specifically Identified As Aluminium(Iii) Oxide.
What is the chemical name of alumina? In this video we'll write the correct name for al2o3. When aluminum and oxygen become an ionic compound the formula is?, answer.